ABOUT 2023 EVENT
What You Missed:
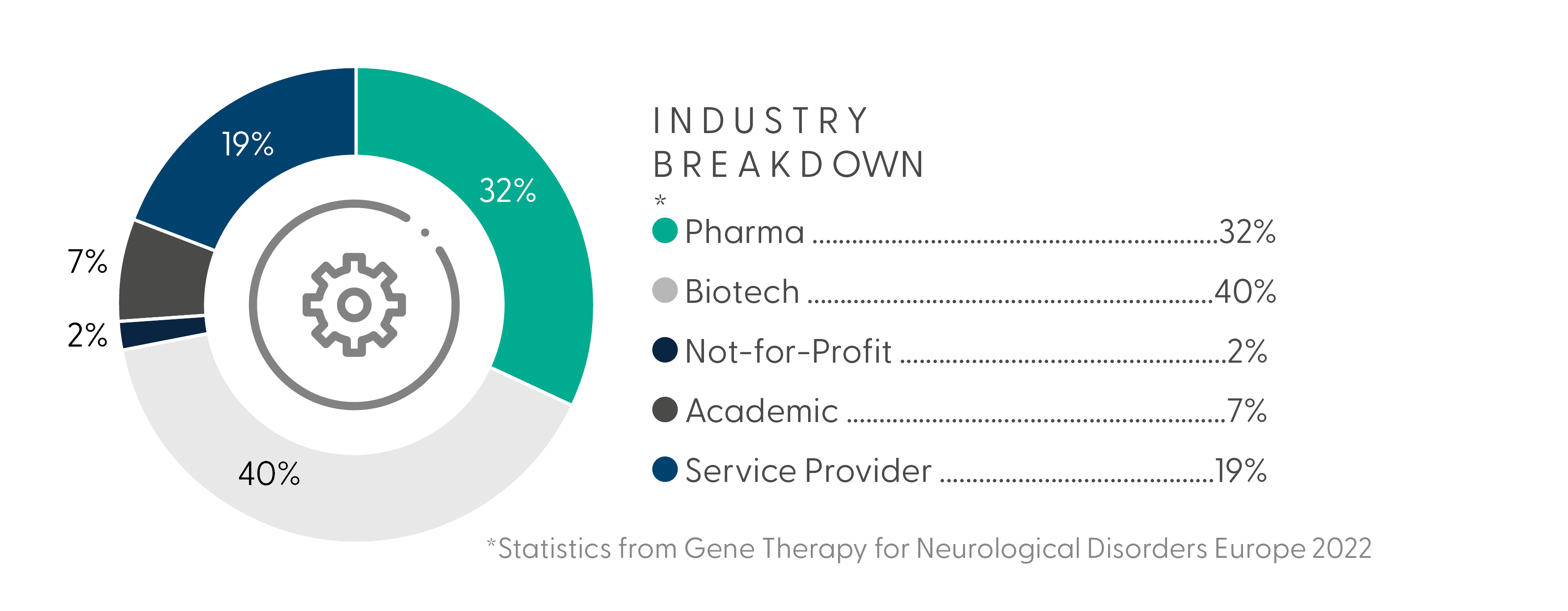
4th Annual Gene Therapy for Neurological Disorders Summit Europe
After a turbulent year for gene therapy, the field is now making a resurgence powered by the approvals of Upstaza by the EMA and Skysona by the FDA.
Additionally, with the majority of new drugs that have been identified in 2022 being for neurological disorders, it is clear that the future for gene therapy is in CNS.
Returning as the pinnacle event for gene therapy for neurological disorders developers in Europe, enabling you to bring your gene therapy to patients in Europe. This year’s content takes a greater focus on:
- Innovation in preclinical models and vector technology
- Showcasing novel approaches to improve delivery in a safe manner
- Optimizing administration route to reduce the patient
- Understanding what needs to be done to crack the European market
Covering both neurodevelopmental and neurodegenerative disorders, join 80+ of your peers in Amsterdam this June. We’ll be welcoming those leading biopharma and academia, working in preclinical, clinical and market access to help get safer, more efficacious gene therapies for neurological disorders to market in Europe.
As the gene therapy community gets a second wind and as the clinical promise in neurology begins to be realised, now is the time to get your hands on the latest innovations in this field to bring safe, efficacious and commercially successful products to market in Europe.
Join your Peers to:
Uncover advancements in pre-clinical models for improved translatability to the clinic to improve the clinical success of your asset with AskBio and the University of Navarra
Learn how to optimise targeting and delivery to the CNS using novel vector technology with Eli Lilly & Co, VectorY and Capsida Biotherapeutics to ensure safe and efficacious delivery across the blood brain barrier for your gene therapies
Explore different delivery routes and devices for reduced patient burden and improved efficacy with Portail Public Hospital of Paris and The University of Cardiff
Overcome disease specific clinical trial challenges like selecting appropriate biomarkers, endpoints, and patient selection with Voyager and UCL to improve the outcomes of your clinical studies
Join our regulatory workshop hosted by Astellas to better understand regional regulatory requirements to strive towards getting your drug approved by the EMA and FDA
Who Will You Meet?
Join 80+ of your peers from leading biopharma and academia including C-level executives, directors and senior scientists, helping to advance safe, efficacious and commercially successful gene therapies to market in Europe including: Vector technology, Delivery, Preclinical modelling and Market access
What Your Peers Have to Say
‘Inspirational and with interacting speakers and networking’ – Casper Gotzsche, Bayer
‘This event was very helpful to better understand current landscape of GT therapies in CNS’ – Frank Simmersbach, Daiichi Sankyo Europe
‘A great opportunity to network within the CNS gene therapy community’ – Sue Brown, Passage Bio

